Describe Rutherford's Model of the Atom
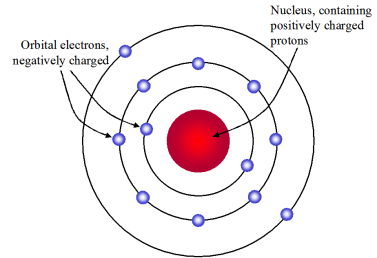
The team concluded that. In Rutherfords model negatively charged electrons surround a dense positively charged nucleus.

Rutherford S Atomic Model Chemistry For Non Majors
The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.

. Through the foil undeflected. Rutherfords atomic model is the model which described the atom as a tiny dense positively charged core called a nucleus in which nearly all the mass is concentrated around which the light negative constituents called electrons circulate at some distance much like planets revolving around the Sun. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.
Ernest Rutherford was a New Zealand born physicist who in 1911 described the structure of an atom which was an improvement on the plum in pudding model of atom Rutherford model is also known as the Rutherford atomic model planetary model of the atom or the nuclear model of the atomThe Rutherford atomic theory has defined the atom as a tiny dense positively charged. In Bohrs model the electrons are assigned to. It is described as follows 1 Atom consists of positively charged small dense nucleus having all the protons and neutrons in it.
The entire mass of atom is concentrated in nucleus. According to the Rutherford atomic model. The gold foil was about 1000 atoms thick.
A Rutherfords model of atom- Ernest Rutherford was interested in knowing how the electrons are arranged within an atom. RUTHERFORDS MODEL OF ATOM model was correct. He also claimed that.
He concluded that all of the positive charge and the majority of the mass of the atom must be concentrated in a very small space in the atoms interior which he called the nucleus. Rutherford directed the GeigerMarsden experiment in 1909 which suggested upon Rutherfords 1911 analysis that J. Rutherfords atomic model became known as the nuclear model.
He called this region of the atom as a nucleus. Rutherfords model of the atom consisted of a positively charged center known as NUCLEUS which also contained most of the atoms mass. Around the nucleus orbited the negatively charged electrons.
According to the Rutherford atomic model. It is like an aquarium with swimming fish representing positive charges. Describe Rutherfords model of the atom and compare it with the model proposed by his student Niels Bohr.
According to Rutherfords model electrons may move anywhere within the volume of space around the nucleus. Arrenhasyd and 3 more users found this answer. Rutherford model states that an atom is composed of a middle core where nearly the whole mass of that atom is determined and light weight particles move around this middle core.
What is Rutherford model of an atom class 9th. This was based upon the bright-line spectra of molecular hydrogen and lead to postulates that describe the electronic structure of the atom as having electrons in discrete energy levels and orbiting the nucleus much like planets orbit a star. Rutherfords Model of the Atom Rutherford proposed that each atom has a dense central core which he called the nucleus The nucleus is a central region that is.
It is described as follows 1 Atom consists of positively charged small dense nucleus having all the protons and neutrons in it. Rutherfords model shows that an atom is mostly empty space with electrons orbiting a fixed positively charged nucleus in set predictable paths. According to the Rutherford model an atom is largely empty space comprising electrons that are negatively charged surrounding the.
The model described the atom as a tiny dense positively charged core called a nucleus in which nearly all the mass is concentrated around which the light negative. The Rutherford model was eventually replaced by the Bohr Concentric Ring Model of the atom. Previous question Next question.
Rutherford model also called Rutherford atomic model nuclear atom or planetary model of the atom description of the structure of atoms proposed 1911 by the New Zealand-born physicist Ernest Rutherford. A stream of high energy a-particles from a radioactive source was directed at a thin foil thickness 100 nm of gold metal. Drawbacks of Rutherford Atomic Model.
Through the foil undeflected. Which statement best describes Rutherfords model of the atom It is like an avocado with the pit representing the nucleus. Thomsons plum pudding model of the atom was incorrect.
He conducted an experiment where he bombarded α-particles in a thin sheet of gold. This model of an atom was developed by Ernest. The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom.
He selected a gold foil because he wanted a layer as thin as possible. Rutherfords Nuclear Model of Atom. By performing an.
ARutherfords model shows that an atom is mostly empty space with electrons orbiting a fixed positively charged nucleus in set predictable paths. In this experiment he studied the trajectory of the α-particles after interaction with the thin sheet of gold. He conducted an experiment in which a thin gold foil was bombarded with fast moving alpha α particles.
Describe Rutherfords model of the atom including the location of protons neutrons and electrons with respect to the nucleus. Rutherford and his students Hans Geiger and Ernest Marsden bombarded very thin gold foil with α-particles. How does this model explain the deflections of a beam of alpha particles aimed at a sheet of gold foil.
A British Physicist Ernest Rutherford proposed a model of the atomic structure known as Rutherfords Model of Atoms. As before Rutherford atomic model was also challenged and questioned by many. ERNEST RUTHERFORDs Model Rutherford shot alpha particles at gold foil.
Rutherford model proposed that the negatively charged electrons surround the nucleus of an. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The nucleus is the tiny dense central core of the atom and is composed of protons and neutrons.
Rutherfords atomic model was based on alpha particle scattering experiment. This model of an atom was developed by Ernest Rutherford. In the nuclear atom the protons and.
Rutherfords famous α-particle scattering experiment is represented in Figure.

Atom Rutherford S Nuclear Model Britannica
Rutherford Model Which Best Describes Rutherford S Model Chemistry

Rutherford Atomic Model Observations And Limitations In Detail

Comments
Post a Comment